A new type of biodegradable ultrasound implant based on piezoelectric nanofibres could improve outcomes for patients with brain cancer.
Researchers led by Thanh Nguyen from the at the University of Connecticut’s department of mechanical engineering fabricated the devices from crystals of glycine, an amino acid found in the human body. Glycine is not only non-toxic and biodegradable, it is also highly piezoelectric, enabling the creation of a powerful ultrasound transducer that could help treat brain tumours.
Brain tumours are particularly difficult to treat because the chemotherapy drugs that would be effective in tackling them are blocked from entering the brain by the blood–brain barrier (BBB). This barrier is a very tight junction of cells lining the blood vessel walls that prevents particles and large molecules from making their way through and damaging the brain. However, ultrasound can be safely used to temporarily alter the shape of the barrier cells such that chemotherapy drugs circulating in the bloodstream can pass through to the brain tissues.
Currently, to achieve such BBB opening requires the use of multiple ultrasound transducers located outside the body, together with very high intensity ultrasound to enable penetration through the thick human skull bone.
“That strong ultrasound can easily damage brain tissues and is not practical for multiple-time applications which are required to repeatedly deliver chemotherapeutics,†Nguyen tells Physics World.
By contrast, the team’s new device would be implanted during the tumour removal surgery, and “can generate a powerful acoustic wave deep inside the brain tissues under a small supplied voltage to open the BBBâ€. The ultrasound would be triggered repeatedly as required to deliver the chemotherapy that kills off the residual cancer cells at tumour sites. After a set period of time following treatment the implant biodegrades, thereby eliminating the need for surgery to remove it.
The research, reported in Science Advances, demonstrated that the team’s device used in conjunction with the chemotherapy drug paclitaxel significantly extended the lifetime of mice with glioblastomas (the most aggressive form of brain tumour) compared with mice receiving the drugs but no ultrasound treatment.
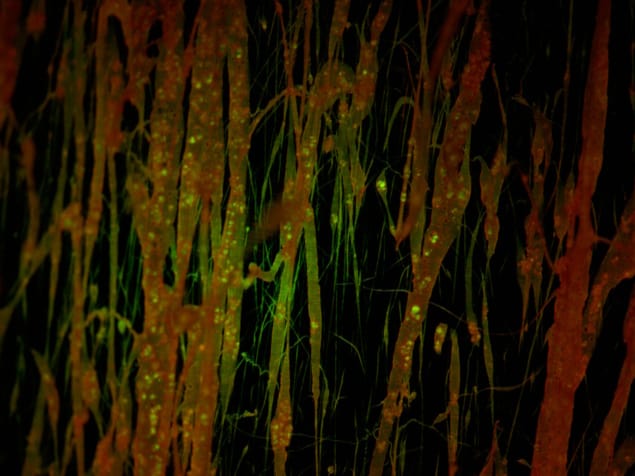
However, there is a catch when making implantable ultrasound devices from glycine crystals. “The crystals are very brittle and highly water soluble, which makes the handling, fabrication and body implantation extremely challenging,†explains Nguyen. To tackle this problem, the team deliberately shattered the crystals into nanoparticles before encapsulating them inside a matrix of the flexible, biodegradable polymer polycaprolactone (PCL).
The researchers created nanofibres of PCL with encapsulated glycine via electrospinning – in which a polymer solution containing the glycine crystals is jetted out and stretched under a high voltage. Unlike conventional solvent-casting techniques, which randomize the crystal domains and dipoles in the crystals and so reduce their piezoelectric output, this processing creates oriented glycine crystals with high piezoelectric performance.

Temporary pacemaker regulates heart rhythm then completely disappears
Next, they made the resulting nanofibres into piezoelectric films and then encased them within a biodegradable polymer that can be tuned to degrade at different rates by varying its thickness or chemistry. Lifetimes ranging from a few days to a few months are achievable, opening up the possibility of the device being useful for a variety of applications. These include enhancing the therapeutic effect of drugs for Alzheimer’s or Parkinson’s disease (which are difficult to get past the BBB), modulating neural signals in the brain, or monitoring brain pressure following traumatic brain injury.
The researchers now plan to test safety and effectiveness in larger animals, while concurrently studying the nanofibre’s piezoelectric and ferroelectric properties to optimize the implant’s performance. Understanding more about these fundamental properties “will enable us to achieve a stable, powerful ultrasound generator with a minimal need of power consumption, minimizing the risk of current leakage and extending the lifetime of the implanted device for broad applications,†says Nguyen.


